
Information Department, PO Box 50005, SE-104 05 Stockholm, Sweden, webbsite: www.kva.se
Tel: +46-8-673 95 95, Fax +46-8-15 56 70, e-mail: info@kva.se
THE NOBEL PRIZE IN CHEMISTRY 1999
- Pressmeddelande på svenska
- Press release in English
- Pressemitteilung auf Deutsch
- Communiqué de presse en français
- Extended version in English (PDF 181kB)
PRESS RELEASE 12 OCTOBER 1999
The Prize I Further reading I The laureate
The Royal Swedish Academy of Sciences has awarded
the 1999 Nobel Prize in Chemistry
to
Professor Ahmed H. Zewail, California Institute of Technology, Pasadena, USA
for showing that it is possible with rapid laser technique to see how
atoms in a molecule move during a chemical reaction.
The Academy's citation:
For his studies of the transition states of chemical reactions using
femtosecond spectroscopy.
This year's laureate in Chemistry is being rewarded for his pioneering investigation of fundamental chemical reactions, using ultra-short laser flashes, on the time scale on which the reactions actually occur. Professor Zewail's contributions have brought about a revolution in chemistry and adjacent sciences, since this type of investigation allows us to understand and predict important reactions.
Development of femtochemistry rewarded
What would a football match on TV be without "slow motion" revealing
afterwards the movements of the players and the ball when a goal is scored?
Chemical reactions are a similar case. The chemists' eagerness to be able
to follow chemical reactions in the greatest detail has prompted increasingly
advanced
technology. This years laureate in Chemistry, Ahmed H. Zewail, has
studied atoms and molecules in "slow motion" during a reaction and seen
what actually happens when chemical bonds break and new ones are created.
Zewail's technique uses what may be described as the world's fastest camera. This uses laser flashes of such short duration that we are down to the time scale on which the reactions actually happen - femtoseconds (fs). One femtosecond is 10-15 seconds, that is, 0.000000000000001 seconds, which is to a second as a second is to 32 million years. This area of physical chemistry has been named femtochemistry.
Femtochemistry enables us to understand why certain chemical reactions
take place but not others. We can also explain why the speed and yield
of reactions depend on temperature. Scientists the world over are studying
processes with femtosecond spectroscopy in gases, in fluids and in solids,
on surfaces and in polymers. Applications range from how catalysts function
and how molecular electronic components must be designed, to the most delicate
mechanisms in life processes and how the medicines of the future should
be produced.
How fast are chemical reactions?
Chemical reactions can, as we all know, take place at very varying
velocities - compare a rusting nail and exploding dynamite! Common to most
reactions is that their velocity increases as temperature rises, i.e. when
molecular motion becomes more violent.
For this reason researchers long believed that a molecule first needs to be activated, 'kicked' over a barrier, if it is to react. When two molecules collide, nothing normally happens, they just bounce apart. But when the temperature is high enough the collision is so violent that they react with one another and new molecules form. Once a molecule has been given a sufficiently strong 'temperature kick' it reacts incredibly fast, whereupon chemical bonds break and new ones form. This also applies to the reactions that appear to be slow (e.g. the rusting nail). The difference is only that the 'temperature kicks' occur more seldom in a slow reaction than in a fast one.
The barrier is determined by the forces that hold atoms together in
the molecule (the chemical bonds) roughly like the gravitational barrier
that a moon rocket from Earth must surmount before it is captured by the
Moon's force field. But until very recently little was known about the
molecule's path up over the barrier and what the molecule really looks
like when it is exactly at the top, its 'transition state'.
Hundred years of research
Svante Arrhenius (Nobel laureate in Chemistry 1903), inspired by van't
Hoff (the first Nobel laureate in Chemistry, 1901) presented just over
a hundred years ago a simple formula for reaction speed as a function of
temperature. But this referred to many molecules at once (macroscopic systems)
and relatively long times. It was not until the 1930s that H. Eyring and
M. Polanyi formulated a theory based on reactions in microscopic systems
of individual molecules. The theoretical assumption was that the transition
state was crossed very rapidly, on the time scale that applies to molecular
vibrations. That it would ever be possible to perform experiments over
such short times was something no-one dreamed of.
But this is exactly what Zewail set out to do. At the end of the 1980s he performed a series of experiments that were to lead to the birth of the research area called femtochemistry. This involves using a high-speed camera to image molecules in the actual course of chemical reactions and trying to capture pictures of them just in the transition state. The camera was based on new laser technology with light flashes of some tens of femtoseconds. The time it takes for the atoms in a molecule to perform one vibration is typically 10-100 fs. That chemical reactions should take place on the same time scale as when the atoms oscillate in the molecules may be compared to two trapeze artists "reacting" with each other on the same time scale as that on which their trapezes swing back and forth.
What did the chemists see as the time resolution was successively improved? The first success was the discovery of substances formed along the way from the original one to the final product, substances termed intermediates. To begin with these were relatively stable molecules or molecule fragments. Each improvement of the time resolution led to new links in a reaction chain, in the form of increasingly short-lived intermediates, being fitted into the puzzle of understanding how the reaction mechanism worked.
The contribution for which Zewail is to receive the Nobel Prize means
that we have reached the end of the road: no chemical reactions take place
faster than this. With femtosecond spectroscopy we can for the first time
observe in 'slow motion' what happens as the reaction barrier is crossed
and hence also understand the mechanistic background to Arrhenius' formula
for temperature dependence and to the formulae for which van't Hoff was
awarded his Nobel Prize.
Femtochemistry in practice
In femtosecond spectroscopy the original substances are mixed as beams
of molecules in a vacuum chamber. An ultrafast laser then injects two pulses:
first a powerful pump pulse that strikes the molecule and excites
it to a higher energy state, and then a weaker probe pulse at a
wavelength chosen to detect the original molecule or an altered form of
this. The pump pulse is the starting signal for the reaction while the
probe pulse examines what is happening. By varying the time interval between
the two pulses it is possible to see how quickly the original molecule
is transformed. The new shapes the molecule takes when it is excited -
perhaps going through one or more transition states - have spectra that
may serve as fingerprints. The time interval between the pulses can be
varied simply by causing the probe pulse to make a detour via mirrors.
Not a long detour: the light covers the distance of 0.03 mm in 100 fs!
To better understand what happens, the fingerprint and the time elapsing
are then compared with theoretical simulations based on results of quantum
chemical calculations (Nobel Prize in Chemistry 1998) of spectra and energies
for the molecules in their various states.
The first experiments
In his first experiments Zewail studied the disintegration of iodocyanide:
ICN -->I + CN. His team were able to observe a
transition state exactly when the I-C bond was about to break: the whole
reaction takes place in 200 femtoseconds.
In another important experiment Zewail studied the dissociation of sodium iodide (NaI): NaI --> Na + I. The pump pulse excites the ion pair Na+ I - which has an equilibrium distance of 2.8 Å between nuclei (Fig. 1) to an activated form [NaI]* which then assumes covalent bonding. However, its properties change when the molecules vibrate; when the nuclei are at their outer turning points, 10-15 Å apart, the electron structure is ionic, while at short distances it is covalent. At a certain point on the vibration cycle, just when the nuclei are 6.9 Å apart, there is a great probability that the molecule will fall back to its ground state or decay into sodium and iodine atoms.
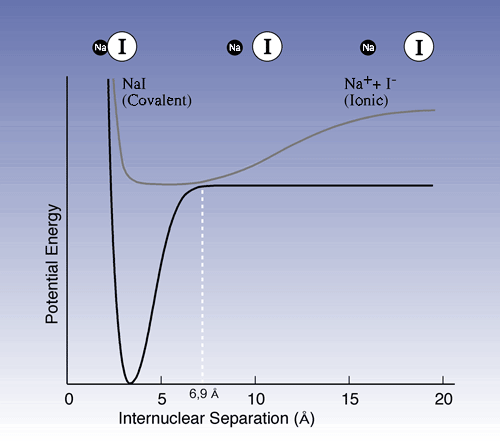
Figure 1
Potential energy curves showing ground state and excited
state for NaI. The upper curve shows the molecule vibrations in excited
NaI. When the distance between the sodium nucleus and the iodine nucleus
is short the covalent bond dominates, while the ion bond dominates at a
greater distance. The vibrations may be compared to those of a marble rolling
back and forth in a dish. As the 6.9 Å point is passed there is a
chance that the marble will roll down to the lower curve. There it may
end up in the pit to the left (return to ground state) or fly out to the
right (decay into sodium and iodine atoms respectively).
Zewail also studied the reaction between hydrogen and carbon dioxide:
H + CO2 --> CO
+ OH a reaction that takes place in the atmosphere and in combustion. He
showed that the reaction crosses a relatively long state of HOCO (1 000
fs).
A question that has occupied many chemists is why certain chemical bonds are more reactive than others and what happens if there are two equivalent bonds in one molecule: will they break simultaneously or one at a time? To answer this kind of question Zewail and his co-workers studied the disassociation of tetrafluordiiodethane (C2I2F4) into tetrafluorethylene (C2F4) and two iodine atoms (I):
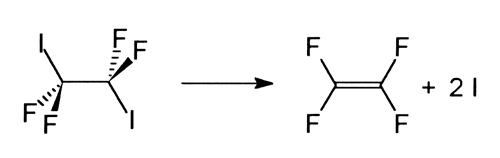
They discovered that the two C-I bonds, despite their equivalence in the original molecule, break one at a time.
Research is extra interesting when the results are unexpected. Zewail
studied what may be thought the simple reaction between benzene, a ring
of six carbon atoms (C6H6)
and iodine (I2), a molecule consisting
of two iodine atoms. When the two molecules become sufficiently close together
they form a complex. The laser flash causes an electron to be shot from
the benzene molecule into the iodine molecule. This then becomes negatively
charged while the benzene molecule becomes positively charged. The negative
and positive charges cause the benzene and the nearest iodine atom to be
rapidly drawn to one another. The bond between the two iodine atoms is
stretched when one of them is sucked in towards the benzene, whereupon
the other atom breaks free and flies away. All this happens within 750
fs. Zewail found, however, that this is not the only way individual iodine
atoms can be formed: sometimes the electron falls back onto benzene. But
it is already too late for the iodine atoms: like a stretched rubber band
breaking, the bond between the two atoms breaks and they fly apart.
Research explosion
A much studied model reaction in organic chemistry is the ring opening
of cyclobutane to yield ethylene or the reverse, the combining of two ethylene
molecules to form cyclobutane. The reaction may thus go directly via one
transition state with a simple activation barrier as shown schematically
on the left in Figure 2. Alternatively, it may proceed through a two-stage
mechanism (right) so that first one bond breaks and tetramethylene is formed
as an intermediate. After crossing another activation barrier the tetramethylene
in turn is converted to the final product. Zewail and his co-workers showed
with femtosecond spectroscopy that the intermediate product was in fact
formed, and had a lifetime of 700 fs.
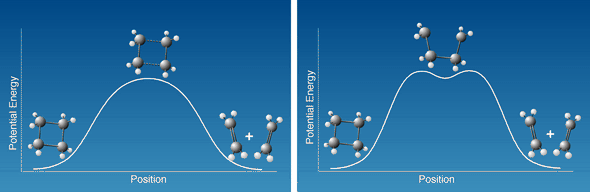
Figure 2
How does the reaction from the cyclobutane molecule
to two ethylene molecules actually proceed? The left-hand figure shows
how the state energy varies if both bonds are stretched and broken simultaneously.
The right-hand figure shows the case where one bond at a time breaks.
Another type of reaction studied with femtosecond technology is the
light-induced conversion of a molecule from one structure to another, photoisomerisation.
The conversion of the stilbene molecule, which includes two benzene
rings, between the cis- and trans- forms was observed by
Zewail and his co-workers.
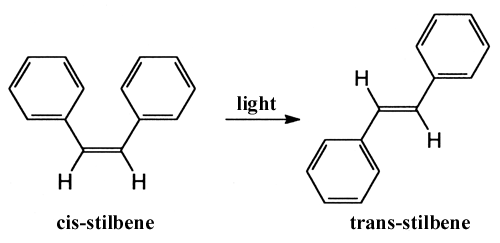
They concluded that during the process the two benzene rings turn synchronously in relation to one another. Similar behaviour has also recently been observed for the retinal molecule, which is the colour substance in rodopsin, the pigment in the rods of the eye. The primary photochemical step, when we perceive light, is a cis-trans conversion around a double bond in retinal. With femtosecond spectroscopy other researchers have found that the process takes 200 fs and that a certain amount of vibration remains in the product of the reaction. The speed of the reaction suggests that energy from the absorbed photon is not first redistributed but is localised directly to the relevant double bond. This would explain the high efficiency (70%) and hence the eye's good night vision. Another biologically important example where femtochemistry has explained efficient energy conversion is in chlorophyll molecules, which capture light in photosynthesis.
Femtosecond studies following Zewail's work are being performed intensively the world over, using not only molecular beams but also processes on surfaces (e.g. to understand and improve catalysts), in liquids and solvents (to understand mechanisms of the dissolving of and reactions between substances in solution) and in polymers (e.g. to develop new material for use in electronics). Another important research field is studies of biological systems. Knowledge of the mechanisms of chemical reactions is also important for our ability to control the reactions. A desired chemical reaction is often accompanied by a series of unwanted, competing reactions that lead to a mixture of products and hence the need for separation and cleansing. If the reaction can be controlled by initiating reactivity in selected bonds, this could be avoided.
Femtochemistry has fundamentally changed our view of chemical reactions. From a phenomenon described in relatively vague metaphors such as 'activation' and 'transition state', we can now see the movements of individual atoms as we imagine them. They are no longer invisible. Here lies the reason why the femtochemistry research initiated by this year's Nobel Laureate has led to explosive development. With the world's fastest camera available, only the imagination sets bounds for new problems to tackle.
*****
Further reading
- "Extended version in English" by Professor Bengt Nordén (Top of page)
- M.A. El-Sayed, I. Tanaka and Y. Molin "Ultrafast Processes in Chemistry and Photobiology" Blackwell Science 1995 306 pp, ISBN 0-86542-893-X.
- S. Pedersen, J.L. Herek and A.H. Zewail "The Validity of the Diradical Hypothesis: Direct Femtosecond Studies of the Transition-State Structures". Science Vol 266 (1994) 1359-1364.
- A.H. Zewail "The Birth of Molecules" Scientific American December 1990 p 40-46.
- V.K. Jain "The World's Fastest Camera" The World and I, October 1995 p 156-163.
- Nobel Symposium: Femtochemistry & Femtobiology: Ultrafast Reaction Dynamics at Atomic-Scale Resolution (Editor: V. Sundström) World Scientific, Singapore 1996.
*****
Ahmed Zewail was born in 1946 in Egypt where he grew up and studied at the University of Alexandria. After continued studies in the U.S.A. he graduated for PhD in 1974 at the University of Pennsylvania. After two years at the University of California at Berkeley he was employed at Caltech where he has the Linus Pauling Chair of Chemical Physics since 1990. Zewail is Egyptian and American citizen.
Professor Ahmed H. Zewail
California Institute of Technology
Arthur Amos Noyes Laboratory of Chemical Physics
Mail Code 127-72
Pasadena, California 91125
USA
The amount of the Nobel Prize Award is SEK 7, 900, 000.